Which of the following molecules are achiral? This question delves into the fascinating realm of molecular chirality, a fundamental concept in chemistry that distinguishes between molecules that exist as mirror images and those that do not. Join us on an enlightening journey as we explore the intricacies of chirality and identify the molecules that lack this intriguing property.
Chirality, derived from the Greek word “cheir” meaning hand, refers to the handedness of molecules. Imagine a pair of gloves: one is a right-handed glove, and the other is a left-handed glove. They are mirror images of each other, but they are not superimposable.
In the molecular world, chirality manifests in a similar fashion. Chiral molecules, like our gloves, exist in two non-superimposable mirror-image forms, known as enantiomers. Achiral molecules, on the other hand, lack this mirror-image relationship and are superimposable on their mirror images.
Definition of Chirality: Which Of The Following Molecules Are Achiral
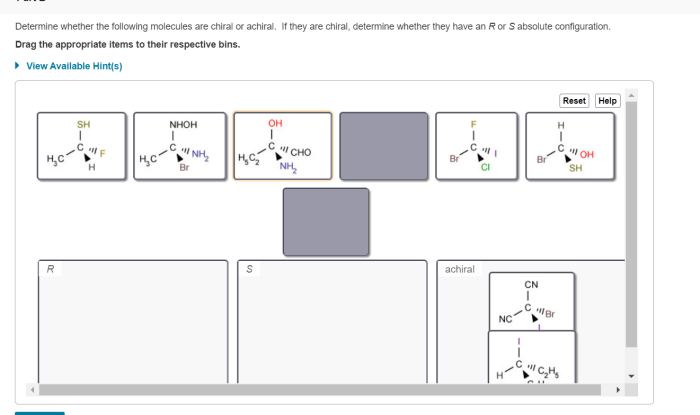
Chirality refers to the property of a molecule that makes it non-superimposable on its mirror image. In other words, a chiral molecule is one that cannot be superimposed on its mirror image, even when rotated or translated.
Chiral molecules exist in two forms, known as enantiomers. Enantiomers are mirror images of each other and have the same chemical and physical properties, except for their interaction with other chiral molecules. This difference in interaction arises from the different spatial arrangement of atoms in enantiomers, which gives them distinct stereochemistry.
Symmetry Elements and Achirality
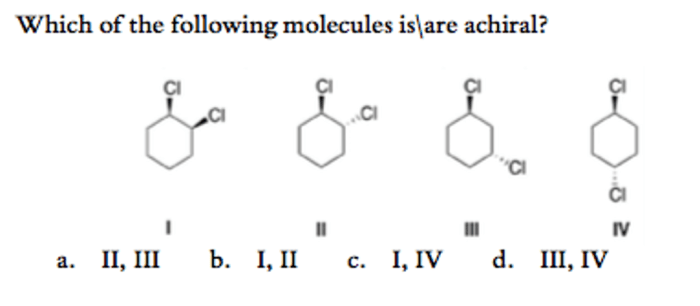
Symmetry elements are geometric features that can be used to determine the chirality of a molecule. These elements include:
- Mirror planes (σ): A mirror plane is a plane that divides a molecule into two mirror-image halves. The presence of a mirror plane indicates that the molecule is achiral.
- Inversion centers (i): An inversion center is a point in a molecule that, when inverted, results in the same molecule. The presence of an inversion center also indicates that the molecule is achiral.
Structural Features Indicating Achirality
Certain structural features can also indicate the achirality of a molecule. These features include:
- Planes of symmetry: A plane of symmetry is a plane that divides a molecule into two identical halves. The presence of a plane of symmetry indicates that the molecule is achiral.
- Identical substituents: If a molecule has two or more identical substituents attached to the same carbon atom, the molecule is achiral.
Methods for Determining Achirality
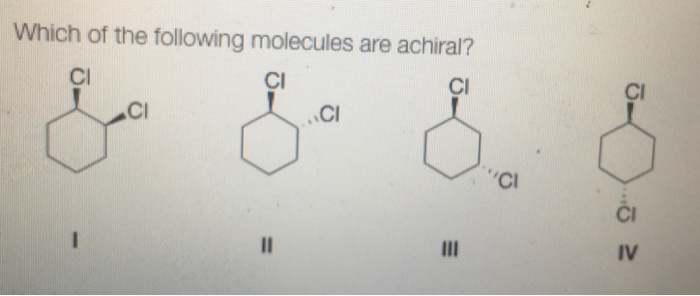
There are several methods that can be used to determine the achirality of a molecule. These methods include:
- Molecular modeling: Molecular modeling software can be used to create a 3D model of a molecule and determine its symmetry. This method can be used to identify mirror planes and inversion centers, which indicate achirality.
- Spectroscopic techniques: Spectroscopic techniques, such as circular dichroism (CD) and optical rotatory dispersion (ORD), can be used to measure the optical activity of a molecule. Optical activity is a measure of the ability of a molecule to rotate plane-polarized light.
Chiral molecules exhibit optical activity, while achiral molecules do not.
Examples of Achiral Molecules
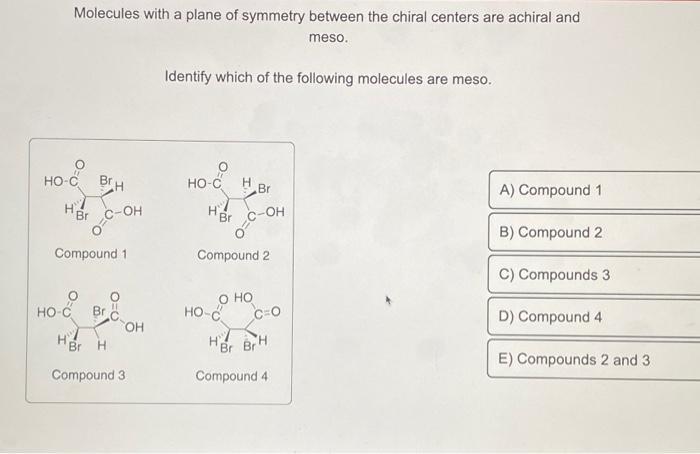
- Methane (CH 4)
- Ethane (C 2H 6)
- Benzene (C 6H 6)
- Carbon dioxide (CO 2)
- Water (H 2O)
FAQ Resource
What is the difference between chiral and achiral molecules?
Chiral molecules exist in two non-superimposable mirror-image forms, known as enantiomers, while achiral molecules are superimposable on their mirror images.
How can I determine if a molecule is achiral?
The presence of symmetry elements, such as mirror planes or inversion centers, can indicate achirality. Additionally, specific structural features, such as symmetry planes or identical substituents, can contribute to the absence of chirality in molecules.
What are some examples of achiral molecules?
Examples of achiral molecules include methane (CH4), carbon dioxide (CO2), and benzene (C6H6).